Marie-Paule Kieny
Chair

Charles Gore
Executive Director
MESSAGE
from the Chair of the Governance Board and Executive Director

«2019 has been the year of three P’s for the Medicines Patent Pool – Progress, Partnerships and Prospects. Today, as the world grapples with an unforeseen pandemic of COVID-19, the work of MPP is as important, if not more so, as it was ten years ago when the organisation was founded.»
MESSAGE
from the Chair of the Governance Board and Executive Director
Reflecting on the year that went by, we bring to you our 2019 Annual Report with its theme, “Supporting Universal Health Coverage through Affordable Medicines.”
Health is a fundamental human right, and we strongly believe that all people, irrespective of who they are or where they live, should be able to access health services they need, without suffering financial hardship. Access to safe, effective, quality and affordable essential medicines everywhere is critical to achieve Universal Health Coverage (UHC). Yet, nearly two billion people, a vast majority living in lowand middle-income countries (LMICs), lack access to essential health products and medicines. Today, as we face the COVID-19 pandemic, equitable access to health innovations wherever you live in the world is more relevant than ever.
The Medicines Patent Pool (MPP), through its voluntary licensing and patent pooling model, works to increase access to affordable lifesaving medicines in LMICs. Since 2010, when MPP was founded, our licences have contributed to furthering the global goals in HIV, hepatitis C and tuberculosis. More recently, we have expanded our remit to essential medicines for diseases beyond HIV, TB and hepatitis C, such as diabetes, cardiovascular disease and cancer, and worked with WHO to identify patented molecules in these new disease areas, where licensing could bring significant public health impact.
2019 has been the year of three P’s for the Medicines Patent Pool - Progress, Partnerships and Prospects.
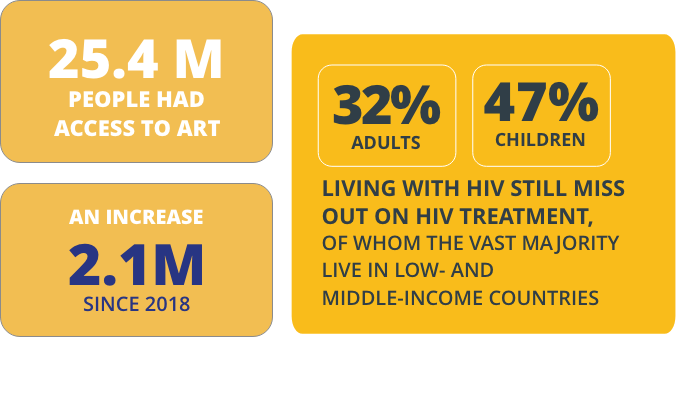
Progress: Between 2012 and 2019, MPP’s work in HIV and hepatitis brought nearly 12 billion doses of treatment to people across 141 countries and generated savings of USD 1.44 billion. In the last six months of 2019 alone, two billion doses of treatment were delivered and USD 210 million were saved. Each dose supplied meant that a life was improved, and each dollar saved meant that more people could be put on treatment.
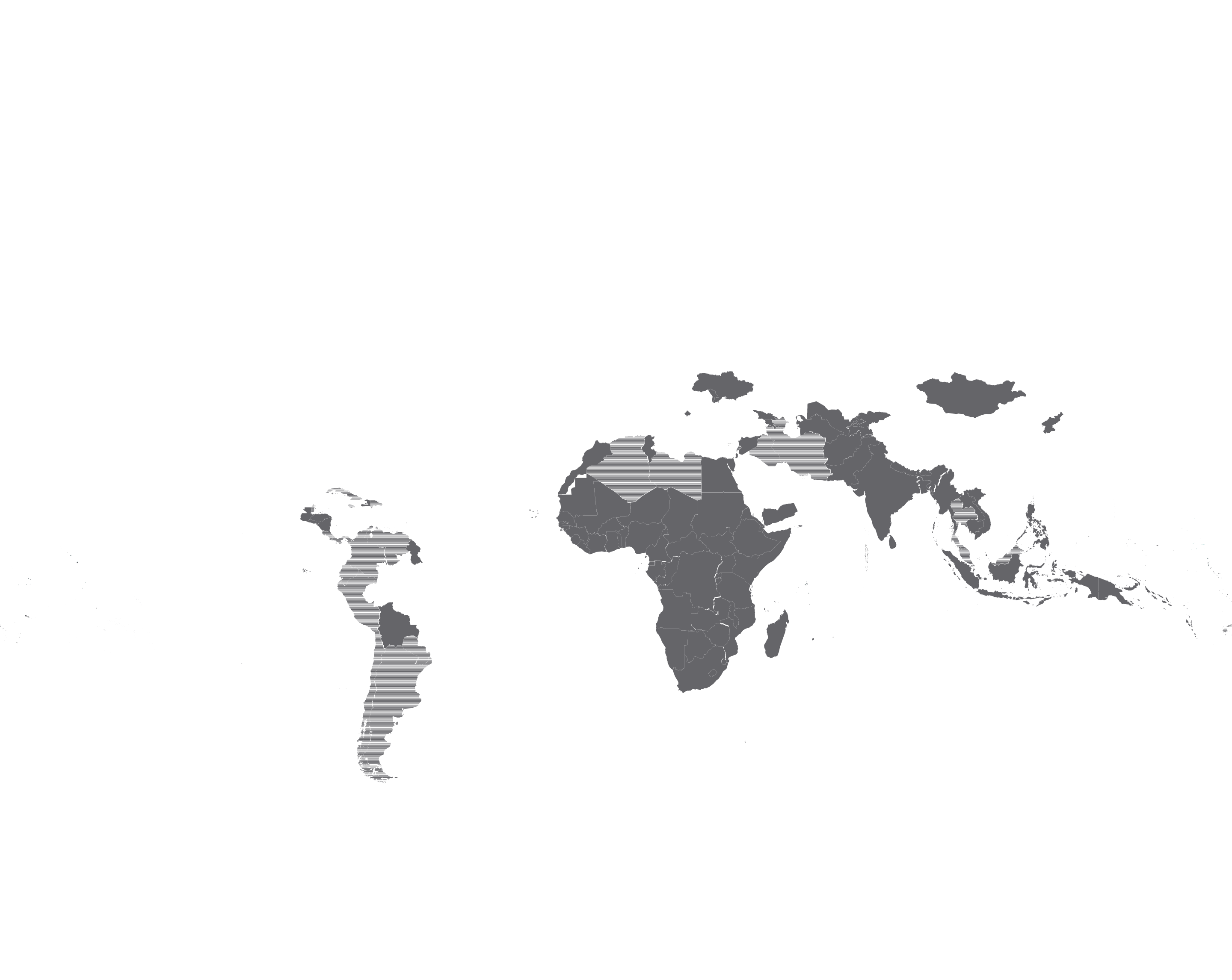
One of our key licences, with ViiV Healthcare, achieved the five-year milestone in 2019. Currently, through the licence 17 generic manufacturers are authorised to produce and sell low-cost single or fixed-dose combination versions of dolutegravir (DTG), WHO’s preferred first-line treatment for people living with HIV (PLHIV). Through these agreements 103 countries where 89% of PLHIV reside are now procuring lowcost and high-quality versions of this safe and effective HIV treatment.
Another area where we have seen good progress is hepatitis C. Today, in most countries, the price of daclatasvir+sofosbuvir (DAC+SOF) treatment is under USD 100, and over 900,000 curative treatments of DAC alone have been supplied through MPP licences since 2016 in 28 countries. Since the end of 2018, MPP also holds the licence for glecaprevir/ pibrentasvir (G/P) from AbbVie that enables qualityassured manufacturers to develop and sell G/P in 96 LMICs. This pan-genotypic regimen, which shortens the treatment duration to just eight weeks, has a unique value in people with kidney disease as well as in children under 12 years and avoids the need for costly genotyping diagnostic tests. It is currently under development by one of our generic partners, Mylan and we hope more manufacturers will enter this space soon.
None of these successes would have been possible without our partners.
Partnerships: Each partner we work with is a critical piece of the access puzzle, which when put together, makes lifesaving medicines accessible for those in need. From the work of an originator, who develops a drug, to us negotiating a licence, to generics developing affordable quality versions of the drug, to organisations like Unitaid that fund development of paediatric formulations for these medicines, to WHO producing guidelines and recommending the drug, to advocates pushing governments to include the drug in their national guidelines, to procurement agencies like PEPFAR and the Global Fund that buy for countries, the list goes on. It is the joint effort of these stakeholders that maximises efficiencies, reduces costs and ultimately achieves the common mission of saving millions of lives. MPP truly values the work of its partners and in this spirit deepened its collaborations further in 2019.
Through the year, we signed a key licence on sutezolid for TB with Pfizer, expanded the reach of existing licences allowing access to millions of more people, ensured sustainable supply through our generic partners, and continued to identify public health needs closely with experts, civil society and countries. By being a part of various consortia, alongside a multitude of partners, including WHO, we contributed to conversations on issues like drug forecasting and access to paediatric formulations.
Prospects: Looking ahead, we developed a prioritisation framework to assess candidate medicines that could play a major role in MPP’s expanded mandate into new disease areas beyond HIV, hepatitis C and TB. Areas where we have begun discussions with relevant stakeholders are diabetes, cancer and cardiovascular disease. 2019 also marked the beginning of MPP’s exploratory journey into making long-acting technologies – for preventing malaria and TB and treating HIV and hepatitis C – accessible to people living in LMICs. Putting access on the agenda from the very start will go a long way in ensuring no country lags behind in obtaining these potentially game-changing technologies.
Today, as the world grapples with an unforeseen pandemic of COVID-19, the work of MPP is as important, if not more so, as it was ten years ago when the organisation was founded. Swiftly realising this, MPP’s Board expanded the organisation’s mandate to COVID-19-related treatments and technologies on 31 March 2020, just days after WHO declared the disease a pandemic. With all that we do, we are striving to leave no one behind. And we thank you for joining us in our efforts.
close

MESSAGE
from the Executive Director a.i. of Unitaid

«When Unitaid founded MPP, many wondered whether the idea of a patent pool for medicines could work. Fast forward ten years, and MPP holds 107 sublicenses with 22 manufacturers and has generated more than USD 1,441 million in savings across a staggering 31 million patients-years of treatment.»
MESSAGE
from the Chair of the Governance Board and Executive Director
In 2019, South Africa announced its switch to a state-of-the-art dolutegravir (DTG)-based HIV regimen that is easier to administer and has fewer side effects. The move will allow one in five people on HIV treatment globally to switch to a simpler, more effective and affordable regimen that also minimizes the development of drug resistance. Behind this milestone is the diligent work of MPP. Its voluntary licensing mechanism complements the joint work by WHO, Unitaid, the Global Fund and PEPFAR in Africa, making it possible for low- and middle-income countries such as South Africa to procure generic versions of DTG.
When Unitaid founded MPP, many wondered whether the idea of a patent pool for medicines could work. Fast forward ten years, and MPP holds 107 sublicenses with 22 manufacturers and has generated more than USD 1.441 billion in savings across a staggering 31 million patient-years of treatment. MPP has also established itself as an authority on patent information through MedsPaL, which provides the latest insights on the licensing status of selected HIV, hepatitis C, tuberculosis and other life-saving medicines in low- and middle-income countries. The platform has become an indispensable resource for a growing number of procurement agencies.
In 2019, MPP’s work was recognized by international forums such as the G7 and the G20, which highlighted its role in improving access to safe, quality medicines that are affordable. These international forums also supported the expansion of MPP’s mandate to the World Health Organization’s (WHO) list of essential medicines as a means of advancing Universal Health Coverage (UHC).
We share MPP’s commitment to promoting UHC and confronting the emerging threat of antimicrobial resistance (AMR), while advancing global goals for major diseases and the 2030 Agenda for Sustainable Development. We are proud to support MPP’s work on HIV, tuberculosis and hepatitis C, and look forward to strengthening our collaboration in the future as new and exciting global health innovations come into being. There is still much to do.
Globally, around four in ten people living with HIV are not accessing antiretroviral treatment, while a mere 20 percent of the people with chronic hepatitis C infection have been diagnosed and only seven percent are treated. On the TB front, more than 95 percent of TB deaths occur in low- and middle-income countries, showing the need to continue developing medicines and tests that are affordable and adapted to the needs of low-resource settings.
In taking stock of the first ten years of MPP, we greet its outstanding achievements and its determination to continue tackling global health challenges – including the COVID-19 pandemic— in collaboration with originators, generic manufacturers and countries. At Unitaid, we remain committed to partnering with MPP to improve and save the lives of millions.
close